Recently, the paper-"Amorphous Iron-Borate Nanolattices as Multifunctional Electrodes for Self-Driven Overall Water Splitting and Rechargeable Zinc-Air Battery”by the researchers in the State Key Laboratory of Heavy Oil of UPC, was published on Small and highlighted in back cover. The corresponding author was Prof. Li Zhongtao and Prof. Wu Mingbo, and the first author was Zhao Weinan.

Facing the increasing energy demand and environmental pollution, it is urgent to promote energy transformation and upgrading, optimize energy structure adjustment and establish a clean and renewable energy system. Among them, electrochemical electrolysis of aquatic hydrogen (2H2O→2H2+O2) has become one of the key links in the clean renewable energy utilization system. However, due to the high potential and slow reaction kinetics of electrolytic water semi-reaction (OER, HER), the current driving voltage of electrolytic water is much higher than the theoretical decomposition potential of water 1.23V. It causes a great waste of energy and increases the application cost, which limits the large-scale application of hydrogen production from electrolytic water. Therefore, the key to the promotion and application of electrolytic aquatic hydrogen production is to synthesize cheap, readily available and highly catalytic electrode materials.
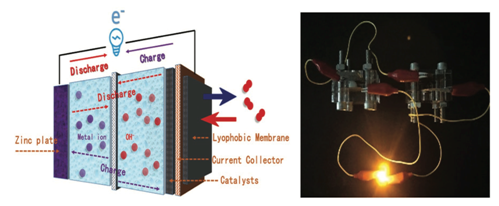
In response to the above challenges, researchers in the State Key Laboratory of Heavy Oil have prepared a novel two-dimensional amorphous iron borate nanosheet catalyst by the solvothermal-self-assembly method. The material has excellent multifunctional electrocatalytic properties in alkaline solution. It has low reaction voltage (1.56V) when catalyzing the total hydrolysis of water and excellent ORR activity when used as the positive electrode of zinc-air battery. DFT theoretical calculations show that the material has lower d-band centers, which can balance the adsorption capacity of active sites to reaction intermediates; amorphous "short-range ordered" two-dimensional structure exposes more active sites, and is easier to precipitate oxygen and hydrogen generated by the reaction, thus speeding up the kinetic process; boric acid with greater electronegativity Roots can attract Fe3+, thus optimizing the overall electron cloud density. Based on the excellent multifunctional electrocatalytic performance of the material, a new device of "self-powered-electrolytic water" was designed and assembled, i.e., two zinc-air batteries in series were assembled with the catalyst to drive the electrolytic water reaction with the same positive and negative electrodes. The Faraday efficiency of electrolytic water was up to 96.3%.
The evaluation experts highly appraised the research results. Compared with other electrode materials, the amorphous iron borate material exhibited unique versatility and better catalytic activity. The prepared multi-functional amorphous iron borate electrode material is expected to be photoelectric catalysis, solar cells, metal-air cells and other related energy. Source catalysis provides experimental and theoretical support.
Small is one traditional TOP magazine in Weily's, which enjoys high reputation and worldwide influence in materials science.
Full text link:
https://onlinelibrary.wiley.com/doi/10.1002/smll.201802829
Translator: Li Zhongtao
Editor: Bu Lingduo
Updated: 2018-12-06